Neuromodulation Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
The Neuromodulation Devices pipeline market research report provides comprehensive information about the Neuromodulation Devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
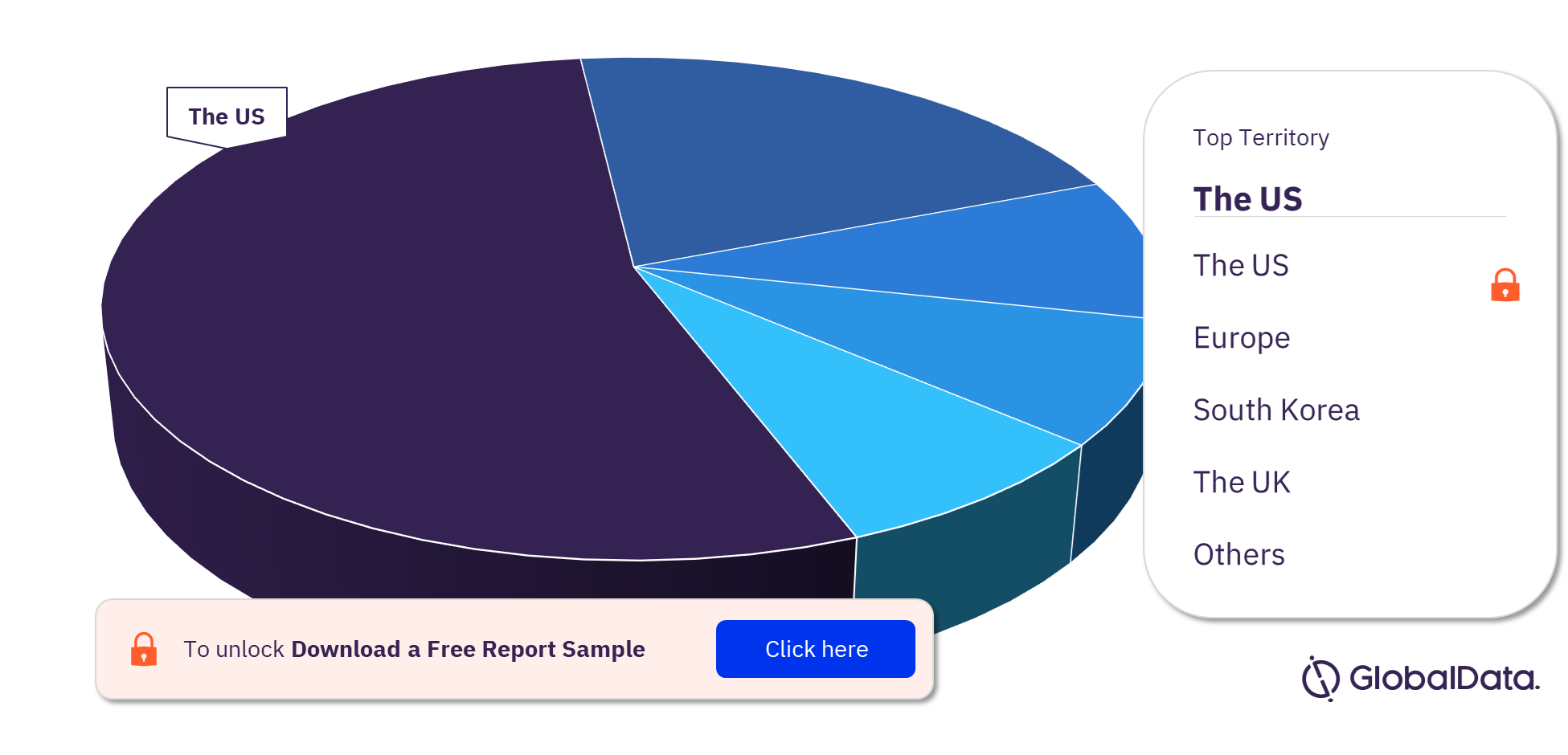
Neuromodulation Devices Pipeline Products Market Segmentation, by Territories
Some of the key territories with products in the pipeline are the US, Europe, South Korea, the UK, Australia China, Israel, Canada, Japan, and New Zealand. As of September 2022, the US has the highest number of products in the pipeline out of them all.
Neuromodulation Devices Pipeline Products Market Analysis, by Territories, 2022 (%)
For more territory insights into the Neuromodulation devices pipeline products market, download a free report sample
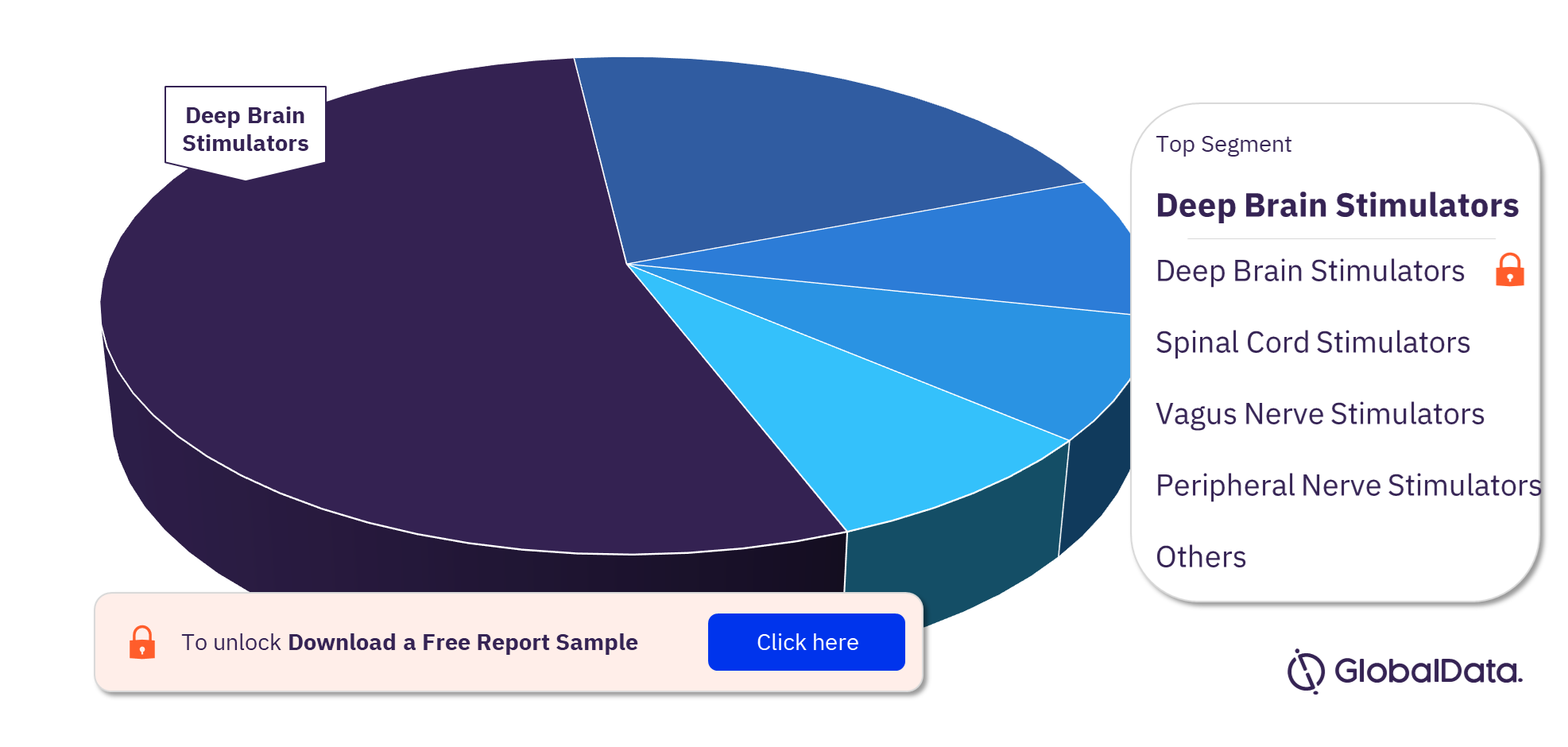
Neuromodulation Devices Pipeline Products Market by Segments
The key segments in the Neuromodulation Devices pipeline products market are Deep Brain Stimulators (DBS), Spinal Cord Stimulators (SCS), Vagus Nerve Stimulators (VNS), Peripheral Nerve Stimulators (PNS), Cortical Stimulators, Gastric Electric Stimulators (GES), Sacral Nerve Stimulators (SNS), and Leads. Deep Brain Stimulators have the greatest number of products in the pipeline.
Neuromodulation Devices Pipeline Products Market Analysis, by Segments, 2022 (%)
For more segment insights into the Neuromodulation devices pipeline products market, download a free report sample
Neuromodulation Devices Pipeline Products Market Segmentation by Key Regulatory Paths
Some of the key regulatory paths followed by the Neuromodulation Devices pipeline products market are PMA, 510(k), CE, TGA, de novo, UKCA, MDITAC, NMPA, HDE Approvals, and MDL. Most of the products follow the PMA pathway to enter the market.
Neuromodulation Devices Pipeline Products Market Analysis, by Regulatory Paths, 2022 (%)
For more Neuromodulation devices pipeline products regulatory path insights, download a free report sample
Competitive Landscape
Some of the leading companies in the Neuromodulation Devices pipeline products market are Aalto University, ab medica spa, Actegy Ltd., Actipulse Neuroscience Inc, Actuated Medical Inc, Adaptive Neuromodulation GmbH, Adept Neuro SA, Adriakaim Inc, Afferent Corporation, and Afferent Technologies.
Aalto University: It is an educational university that offers master’s degrees, doctoral degrees, graduate, postgraduate, and other programs. The university offers technology transfer and licensing to deploy its patented technologies in practice and carries out a wide variety of joint activities with companies and other partners. It offers services to students from Finland and other countries. Aalto University is headquartered in Espoo, Greater Helsinki, Finland.
AB Medica SpA: It is a medical technology company that offers robotic solutions. AB Medica offers services such as consultancy, assistance and training, surgical console, assembly and testing of medical devices, and others. The company partners with universities, research centers, and scientific laboratories. It has operations in Rome, Padova, and Fisciano. AB Medica is headquartered in Milan, Italy.
Actuated Medical Inc: It is a medical device company that develops minimally invasive instruments. The company provides products such as controlled tissue penetration systems, occlusion clearing systems, and MRI-compatible systems. It operates through a manufacturing facility in Bellefonte. Actuated Medical is headquartered in Bellefonte, Pennsylvania, the US.
Neuromodulation Devices Pipeline Products Market Report Overview
| Key Territories | The US, Europe, South Korea, the UK, Australia China, Israel, Canada, Japan, and New Zealand |
| Key Segments | Deep Brain Stimulators (DBS), Spinal Cord Stimulators (SCS), Vagus Nerve Stimulators (VNS), Peripheral Nerve Stimulators (PNS), Cortical Stimulators, Gastric Electric Stimulators (GES), Sacral Nerve Stimulators (SNS), and Leads |
| Key Regulatory Paths | PMA, 510(k), CE, TGA, de novo, UKCA, MDITAC, NMPA, HDE Approvals, and MDL |
| Leading Companies | Aalto University, ab medica spa, Actegy Ltd., Actipulse Neuroscience Inc, Actuated Medical Inc, Adaptive Neuromodulation GmbH, Adept Neuro SA, Adriakaim Inc, Afferent Corporation, and Afferent Technologies |
Segments Covered in the Report
Neuromodulation Devices Pipeline Products Market Territories Outlook
- The US
- Europe
- South Korea
- the UK
- Australia China
- Israel
- Canada
- Japan
- New Zealand
Neuromodulation Devices Pipeline Products Market Segments Outlook
- Deep Brain Stimulators (DBS)
- Spinal Cord Stimulators (SCS)
- Vagus Nerve Stimulators (VNS)
- Peripheral Nerve Stimulators (PNS)
- Cortical Stimulators
- Gastric Electric Stimulators (GES)
- Sacral Nerve Stimulators (SNS)
- Leads
Scope
This report provides:
- Extensive coverage of the Neuromodulation Devices under development.
- Details of major pipeline products which include product description, licensing, collaboration details, and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates.
- Reviews of the major players involved in the development of Neuromodulation Devices and lists all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from early development to the approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent Developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with a potentially strong product portfolio and create effective counterstrategies to gain a competitive advantage.
- Identify and understand important and diverse types of Neuromodulation Devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
ab medica spa
Actegy Ltd.
Actipulse Neuroscience Inc
Actuated Medical Inc
Adaptive Neuromodulation GmbH
Adept Neuro SA
Adriakaim Inc
Afferent Corporation
Afferent Technologies
Aleva Neurotherapeutics SA
Alfa Rhythm Ltd
Allegheny Health Network
Allevion Therapeutics Inc
Apnex Medical Inc
Attune Neurosciences Inc
Aucta Technologies Inc
AURIMOD GmbH
Avation Medical
Axilum Robotics
Axonic SAS
Axonics Inc
Barinetics Corporation
Battelle Memorial Institute
Baylor College of Medicine
BBB Technologies Inc
Beijing Lingchuang Yigu Technology Development Co Ltd
Beta-Stim LTD
BioElectric Solutions Inc.
Bioinduction Limited
Bioness Inc
Biophysical Mind Technologies Ltd.
Biospark Technologies Pte Ltd
Biotronik AG
Biotronik Inc
Biotronik SE & Co KG
BlueWind Medical Ltd
Boston Children's Hospital
Boston Scientific Corp
BrainsGate Ltd
Brainsway Ltd
Brazos Biomedical LLC
Brown University
Cadence Neuroscience Inc
Cala Health Inc
Callitas Therapeutics Inc
Cardionomic Inc
Carnegie Mellon University
Catalan Institute of Nanoscience and Nanotechnology
Cefaly Technology sprl
Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine
CerboMed GmbH
Cerebain Biotech Corp
Cerephex Corporation
Cerevast Therapeutics Inc
Charco Neurotech Ltd
Checkpoint Surgical Inc
Chordate Medical AB
Cibiem, Inc.
Cirtec Medical Corp
ClearPoint Medical
ClearPoint Neuro Inc
Cleveland Clinic
Cleveland Medical Devices Inc
Clinatec
CNX Medical Inc
Cognito Therapeutics Inc
Coloplast A/S
Columbia Engineering
Columbia University
Comphya SA
Conservocare, LLC
Coridea, LLC
Cortec GmbH
Cortivis
Cyberonics Inc
Deep Brain Innovations, LLC
Deep Brain Stimulation Technologies Pty Ltd
DEYMED Diagnostic
Dignify Therapeutics LLC
Direct Spinal Therapeutics, Inc.
Drexel University
Duke University
DuoCure Ltd.
Dysphagia
EBS Technologies GmbH
EBT Medical Inc
Ecole Polytechnique Federale de Lausanne
Eisana Corp
ElectroCore Inc
Elira Therapeutics Inc
EMKinetics, Inc.
Emory University School of Medicine
Endonovo Therapeutics Inc
EndoStim Inc
Enlight Medical Technologies (Shanghai) Co Ltd
Enopace Biomedical Ltd
Enspire DBS Therapy Inc
EPIC Neuro Inc
Epineuron Technologies Inc
EstimME Ltd.
Evonik Corp
Evren Technologies Inc
Exsurgo Ltd
Feelmore Labs Inc
FemPulse LLC
Fisher Wallace Laboratories, LLC
Flint Rehabilitation
Florida International University
Flow Neuroscience AB
FRD Accel LLC
Functional Neuromodulation Inc.
Futurecure Health Pvt Ltd
G6 Technologies Corporation
Galvani Bioelectronics Ltd
Georgia Institute of Technology
Ghoonuts Co Ltd
GiMer Medical
Gondola Medical Technologies SA
GrayMatters Health Ltd
GTX Medical BV
Helius Medical Technologies Inc
Highland Instruments Inc
Imperial College London
ImThera Medical Inc
INBRAIN Neuroelectronics
InCube Labs LLC
Inner Cosmos Inc
InnoSphere Ltd
Inspire Medical Systems Inc
Integer Holdings Corp
Iota Biosciences Inc
Iowa State University
JelikaLite LLC
Johns Hopkins University
Juno Biomedical Inc
King's College London
Koc University
Koninklijke Philips NV
Lawrence Livermore National Laboratory
Lawson Health Research Institute
Lehigh University
Leptos Biomedical, Inc.
LivaNova PLC
Lockheed Martin Aculight Corporation
Louis Stokes Cleveland VA Medical Center
Louisiana State University
Lynx Design, Inc.
Magnus Medical Inc
MagVenture A/S
Massachusetts Eye and Ear Infirmary
Massachusetts General Hospital
Massachusetts Institute of Technology
Mayo Clinic
MDCN Technologies Inc
MDCN Technology Co Ltd
Meagan Medical Inc.
MedAutonomic Inc
Meddynamics Ltd.
Medical University of South Carolina
Medtrode Inc
Medtronic Inc
Medtronic Plc
Medtronic Xomed Inc
MetaCure Germany GmbH
Metavention Inc
Micro-Leads Inc
MicroTransponder Inc
Monash University
Morari LLC
Motility Medical
MR-Link LLC
Myant Inc
Nagoya University Graduate School of Medicine
National Cheng-Kung University Hospital
National University of Ireland Galway
NDI Medical LLC
Nemechek Technologies LLC
Neogenesis Technologies LLC
Nervive Inc
NeuraStasis Inc
Neuro Device Group SA
Neuro Devices, Inc.
Neurodan A/S
Neuroelectrics Barcelona SLU
NeuroEM Therapeutics Inc
Neurolief Ltd.
NeuroMEDx
NeuroMetrix Inc
Neuromod Devices Ltd
Neuronetics Inc
Neuronix Ltd
Neuronoff Inc
NeuroOne Medical Technologies Corp
NeuroPace Inc
Neuroparticle Corp
NeuroPrex Inc
NeuroPro Technologies Inc
NeuroQore, Inc.
Neuros Medical Inc
Neurosigma Inc
Neurostream Technologies G.P. (Inactive)
NeuroTek Medical Inc
Neurotherapeutic Solutions Ltd
NeuroTronik Inc
Neurovalens Ltd
NeuSpera Medical Inc
Neuvotion Inc
Nevro Corp
New Jersey Institute of Technology
Newronika s.r.l.
Nexeon Medsystems Belgium SPRL
Nexeon MedSystems Inc
Nexstim Plc
Nia Therapeutics Inc
Nihon Kohden Corp
Noctrix Health Inc
Northwestern University
Novo Medical Corporation
Nu Eyne Co Ltd
NuroRestore, Inc.
Nuvectra Corp
NYU Langone Health System
Nyxoah SA
Ohio State University
Olympic Ophthalmics Inc
Onward Technologies Ltd
Otto Bock HealthCare GmbH
Parasym Ltd
PathMaker Neurosystems Inc
Phagenesis Ltd
Polytechnique Montreal
Precisis AG
Presidio Medical Inc
Pulsetto UAB
Pulsus Medical LLC (Inactive)
Purdue University
Quantum Nanostim LLC
QV Bioelectronics Ltd
Ratner BioMedical Inc
Realeve LLC
Remedee Labs SAS
ReShape Lifesciences Inc
Rice University
Ripple
Rowan University
Saluda Medical Pty Ltd
Salvia Bioelectronics BV
San Francisco VA Medical Center
Sapiens
Seraya Medical LLC
Shanghai Keku Medical Technology Co Ltd
ShiraTronics Inc
Silere Medical Technology, Inc. (Inactive)
Soin Neuroscience Inc
Sooma Oy
Soterix Medical Inc
Spark Biomedical Inc
SpineX
SPR Therapeutics LLC
Sree Chitra Tirunal Institute for Medical Sciences & Technology
St. Jude Medical LLC
Stanford University
Stimdia Medical Inc
StimRelieve LLC
Sunnybrook Health Sciences Centre
SUNY Downstate Medical Center
Synchron Inc
SynerFuse Inc
Synergia Medical SA
Tal Medical, Inc.
Teliatry Inc
Texas A&M University
The Alfred Mann Foundation
The Charles Stark Draper Laboratory Inc
The Feinstein Institute for Medical Research
The Magstim Co Ltd
TheraNova LLC
Thync Inc
Trifectas Medical Corp.
Trinity College Dublin
Tristan Technologies Inc
University of Alabama
University of Auckland
University of California Irvine
University of California Los Angeles
University of California San Diego
University of California San Francisco
University of Cambridge
University of Central Florida
University of Colorado
University of Florida
University of Gothenburg
University of Gottingen
University of Helsinki
University of Houston
University of Illinois
University of Kansas
University of Michigan
University of Milan
University of Minnesota
University of North Carolina at Chapel Hill
University of Oxford
University of Pittsburgh
University of Pittsburgh School of Medicine
University of Queensland
University of Rochester
University of South Carolina
University of South Florida
University of Southern California
University of Technology Sydney
University of Texas Health Science Center at Houston
University of Texas Southwestern Medical Center
University of Utah
University of Washington
University of Wisconsin Madison
University of Wollongong
University of Zurich
Uro Medical Corp
Valencia Technologies Corp
Vanderbilt University
Vanderbilt University Medical Center
Virginia Commonwealth University
Wake Forest Baptist Medical Center
Washington University in St Louis
Washington University School of Medicine
Wave Neuroscience Inc
Wavegate Corp
Wayne State University
Wedge Therapeutics
Weizmann Institute of Science
Wisconsin Institute of Nuclear Systems
WISE s.r.l.
Xavant Technology (Pty) Ltd
XN Health Inc
YBrain Inc
Zennea Technologies
Zygood LLC
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key territories in the Neuromodulation devices pipeline products market?
The US, Europe, South Korea, the UK, Australia China, Israel, Canada, Japan, and New Zealand are some of the key territories with products in the pipeline.
-
What are the key segments in the Neuromodulation devices pipeline products market?
The key segments in the Neuromodulation devices pipeline products market are Deep Brain Stimulators (DBS), Spinal Cord Stimulators (SCS), Vagus Nerve Stimulators (VNS), Peripheral Nerve Stimulators (PNS), Cortical Stimulators, Gastric Electric Stimulators (GES), Sacral Nerve Stimulators (SNS), and Leads.
-
What are the key regulatory paths of the Neuromodulation devices pipeline products market?
Some of the key regulatory paths followed by the Neuromodulation devices pipeline products market are PMA, 510(k), CE, TGA, de novo, UKCA, MDITAC, NMPA, HDE Approvals, and MDL.
-
What are the leading companies in the Neuromodulation devices pipeline products market?
Some of the leading companies in the Neuromodulation devices pipeline products market are Aalto University, ab medica spa, Actegy Ltd., Actipulse Neuroscience Inc, Actuated Medical Inc, Adaptive Neuromodulation GmbH, Adept Neuro SA, Adriakaim Inc, Afferent Corporation, and Afferent Technologies.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Neurology Devices reports